Objectives
The ICMR’s initiative to support Phase I clinical trials in India had two main objectives:
- Establishing Phase I Clinical Trial capacity
- Funding the leads that have National Health Priority and Readiness for Phase I Clinical Trial.
The need to build capacity for early-phase clinical trials in India was first identified at a meeting held in 2021 by the Cabinet Secretary of India. This paved the way to building the nation’s first network to conduct early phase clinical trials in order to bridge the gap between pre-clinical research and late phase clinical trials to make India self-reliant as well as the preferred destination for drug development.
The ICMR’s initiative to support Phase I clinical trials in India had two main objectives:
Associate Professor, Scientific Officer F, Dept. of Clinical Pharmacology, ACTREC, Tata Memorial Centre, Navi Mumbai-410210, Maharashtra, India
Professor & Head - Department of Clinical Pharmacology
KEMH&GSMC
Mumbai - 400012, Maharashtra, India
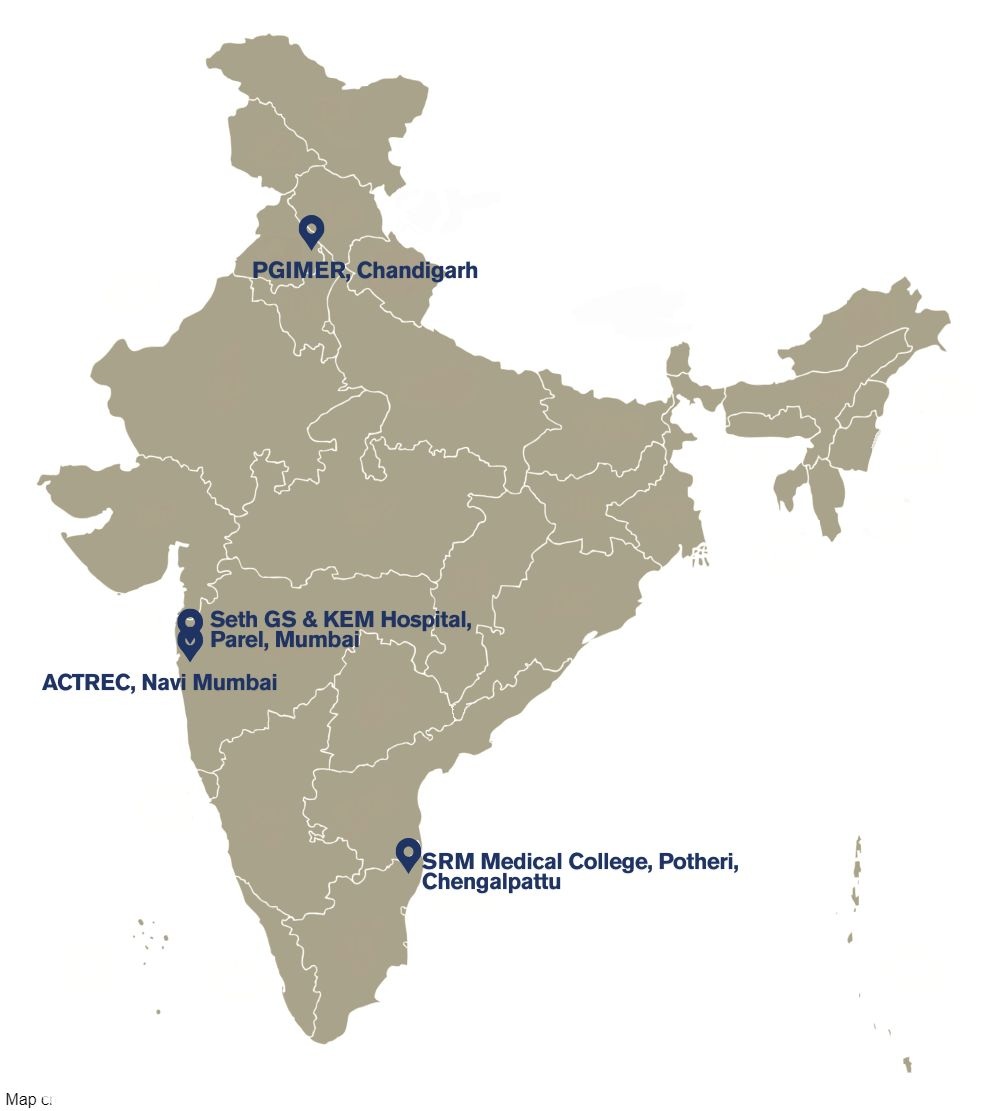
Professor, Clinical Pharmacology Unit,
Department of Pharmacology,
Postgraduate Institute of Medical Education and Research
Chandigarh-160012, India
Head,Professor,Department of Pharmacology,
Centre For Clinical Trials
& Research,
SRM Medical College Hospital
& Research Centre, SRM Nagar, SRM Institute of Science & Technology
Kattankulathur-603203
Tamil Nadu, India.