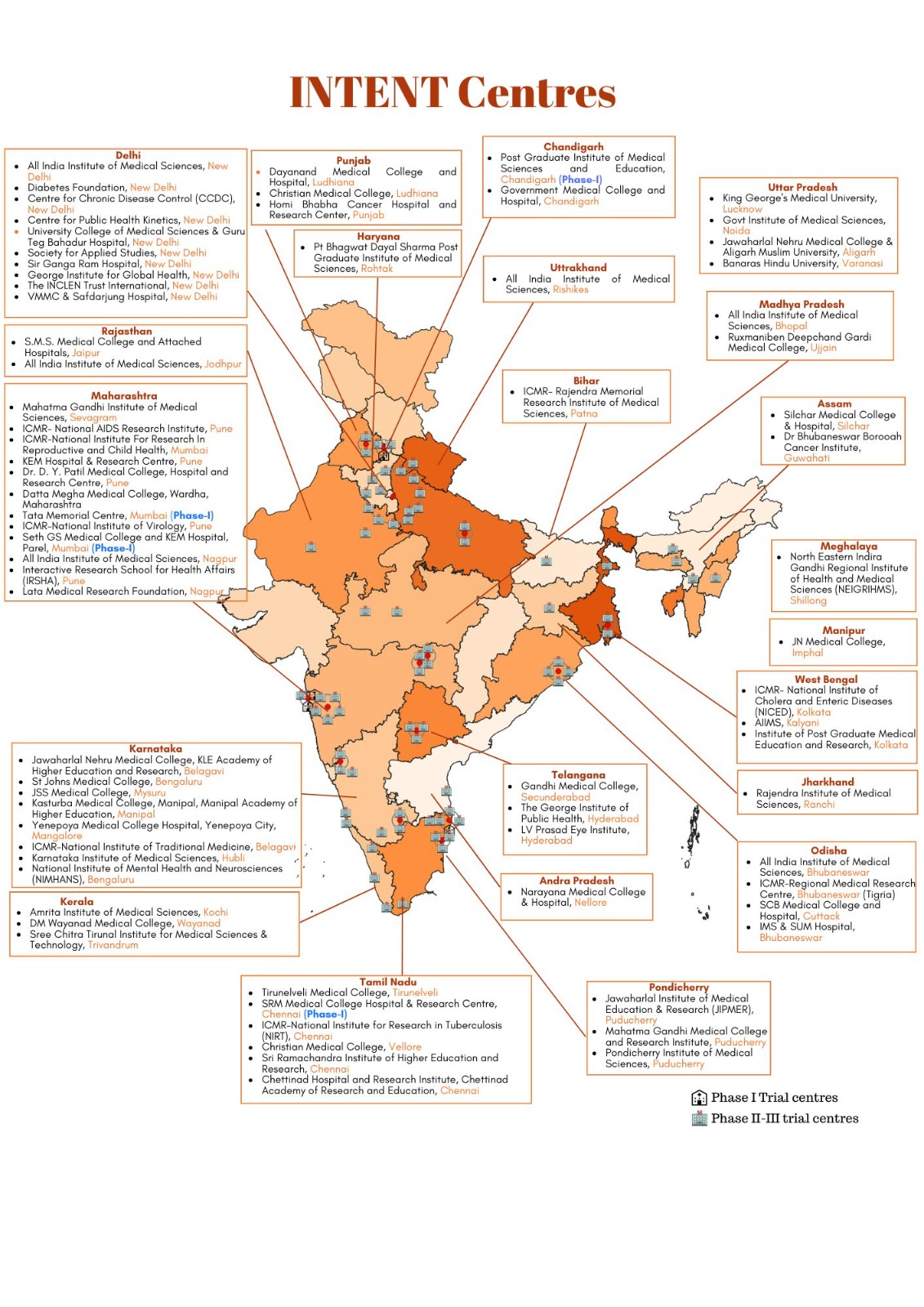
Existing gaps
- India with 18% of the global population conducts only 1·4% of global clinical trials.
- Regulatory clinical trial capacity traditionally exist with pharmaceutical industry and CROs.
- Individuals are frequently sceptic to participate.
- Academic centres lack critical capacity in areas such as regulatory compliance, data management, etc. and restrict themselves to Non-regulatory trials.